19+ rydberg formula calculator
λ vac R1n 1 2 - 1n 2 2 Where λ vac Wavelength of electromagnetic radiation emitted in vacuum R Rydberg Constantapproximately 1097 x 10 7 m-1 n 1 n 2 Integers. Along with the successive Bohrs model Rydberg.

Systematic Study Of Aluminum Nanoclusters And Anion Adsorbates Inorganic Chemistry
1λ 1094710 7 13 2 14 2 1λ 1094710 7 7144 1λ 0053210 7.

. Use our online rydberg equation calculator tool to calculate the wavelength of the light. The Rydbergs Equation is used to determine the wavelength of light emitted by an electron moving between the energy levels of an atom is calculated using Wave Number of Particle. The Rydberg formula is a mathematical formula used to predict the wavelength of light resulting from an electron moving between energy levels of an atom.
C Speed of light 3 108 ms. It is a wavenumber association with the electromagnetic spectrum of each element. Where is the wavelength of electromagnetic radiation emitted in vacuum.
Is the principal quantum number of an. Rydberg Equation Calculator. λ mn 1R μm e Z 2 1n 2 -1m 2 Where λ mn Rydberg Formula R Rydberg.
R Rydberg Constant 1097x 107 m-1. Substituting the inputs we get the following equation for Wavelength of Rydberg Equation. The Balmer-Rydberg Equation calculator computes the wavelength corresponding to the hydrogen atoms energy level differences when an electric current is passed through hydrogen.
Rydberg Constant Formula. λ Wavelength of the emmited light electromagnetic rediation in the vacuum. According to Rydbergs formula.
Calculate the rydberg formula for all hydrogenic elements using this physics calculator. The Rydbergs Equation for hydrogen is used to determine the wavelength of light emitted by an electron moving between the energy levels of an atom with an atomic number of hydrogen. Is the Rydberg constant for hydrogen approximately 1096 775 83 10 7 m 1.
Rydberg formula predicts the wavelength of light. R Rydberg Constant 1097x 107 m-1. Z Number of proton in the nucleus of.
λ Wavelength of the emmited light electromagnetic rediation in the vacuum. The Rydberg formula is a mathematical formula for calculating the wavelength of light emitted by an electron moving between the energy levels of an atom. N1 and n2 are integers.
H c λ h c λ 2176 10 19 1 n 1 2 1 n 2 2 Where h Plancks constant 66 10-34. Z Number of proton in the nucleus of.

Color Cr Intensities Of The Lno Dr Channel In Mg Like Zn Xix Zn 19 Download Scientific Diagram
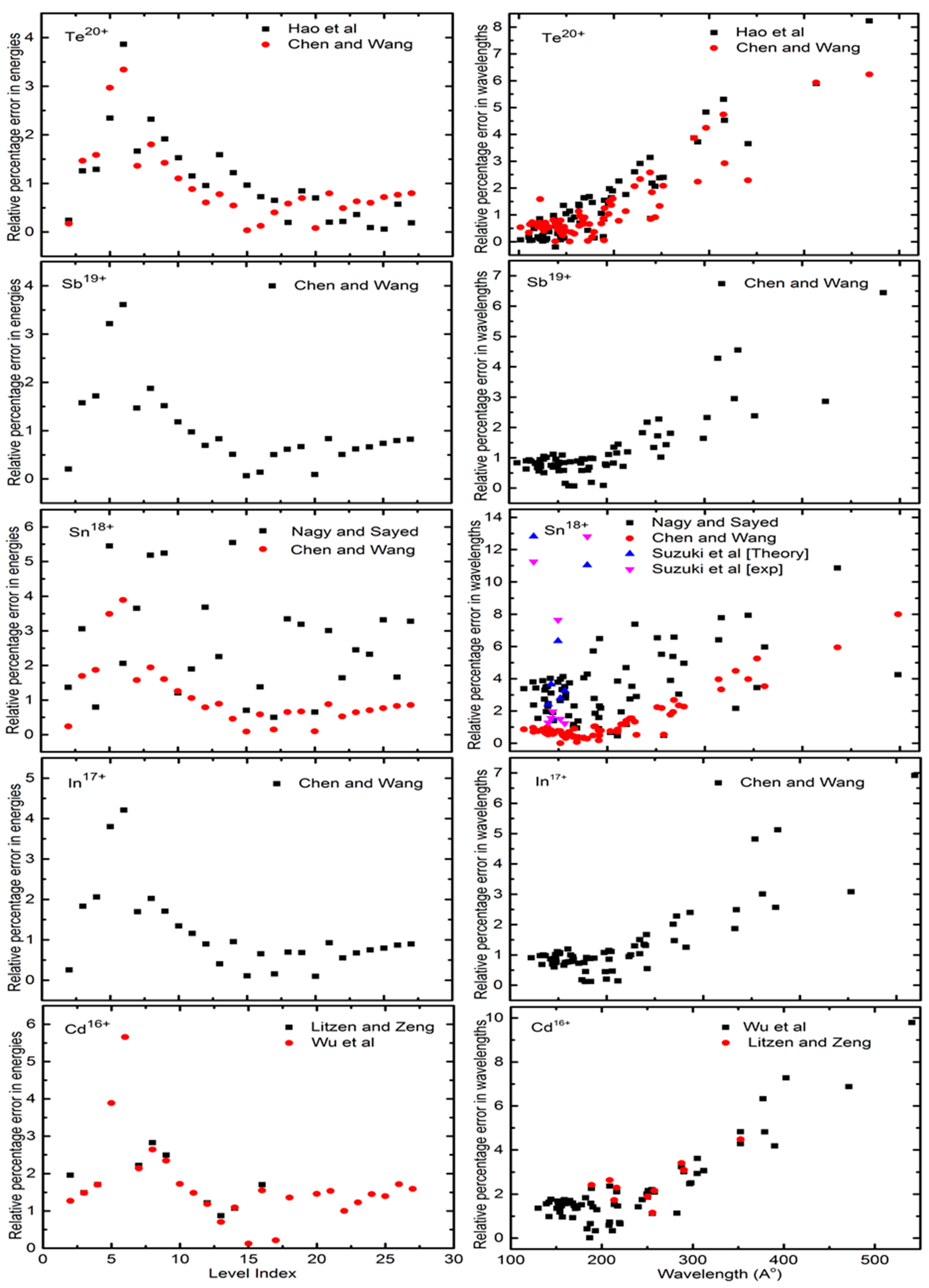
Atoms Free Full Text Electron Impact Excitation Of Ge Like Te20 Ndash Cd16 Ions Html

Rydberg Example Problem Youtube

Using Rydberg S Formula Youtube
Suppose That An Electron Starts In The N 4 Shell Of A Neutral Hydrogen Atom How Many Photons Will Be Emitted Once It Has Fallen To The N 1 Shell Quora

The Ionization Energy Of The Ground State Of Hydrogen Atom Is 2 18 10 18 J The Energy Of An Electron In Its Second Orbit Would Be

Rydberg Equation Calculator Free Calculator To Find Rydberg Equation

Calculate Absorption Rydberg Youtube

Smithells Metals Reference Book 7e Pdf Alloy Metals
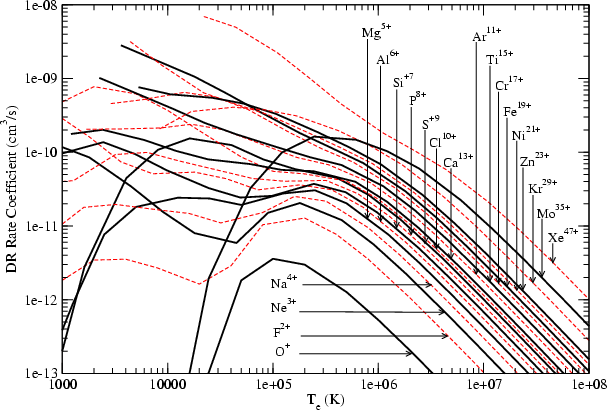
Astronomy Astrophysics
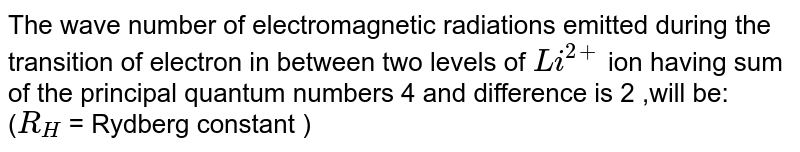
Calculate The Wavelength Emitted During The Transition Of An Electron In Between Two Level Of Li 2 Ion Whose Sum Is 4 And Difference Is 2
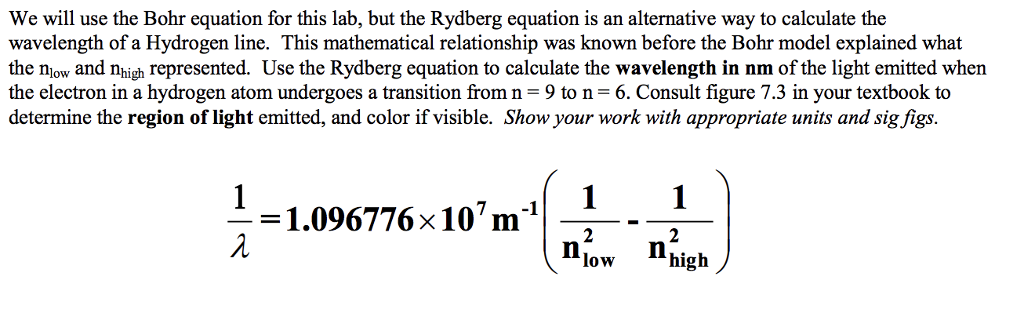
Solved We Will Use The Bohr Equation For This Lab But The Chegg Com

Rydberg Equation Calculator Calculator Academy

Isoelectronic Sequence An Overview Sciencedirect Topics
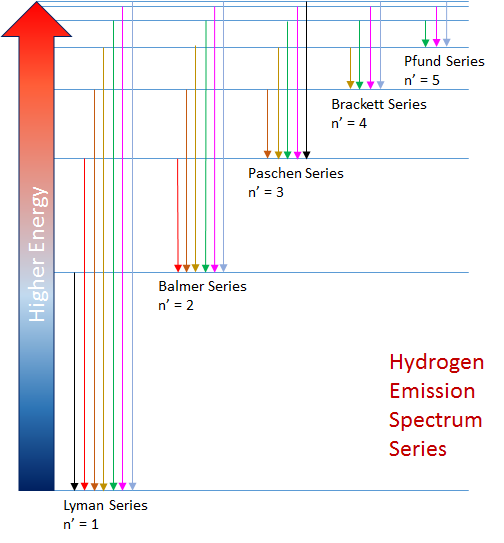
Balmer Rydberg Equation
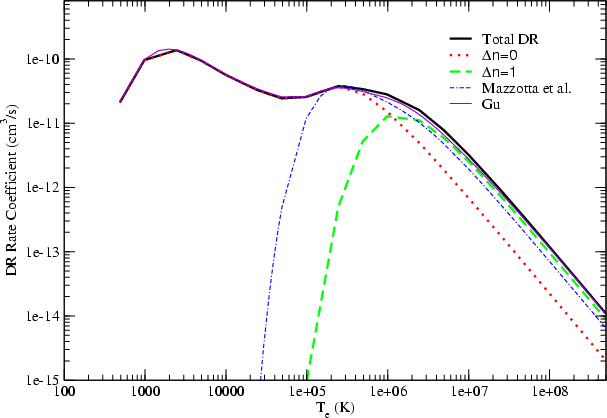
Astronomy Astrophysics
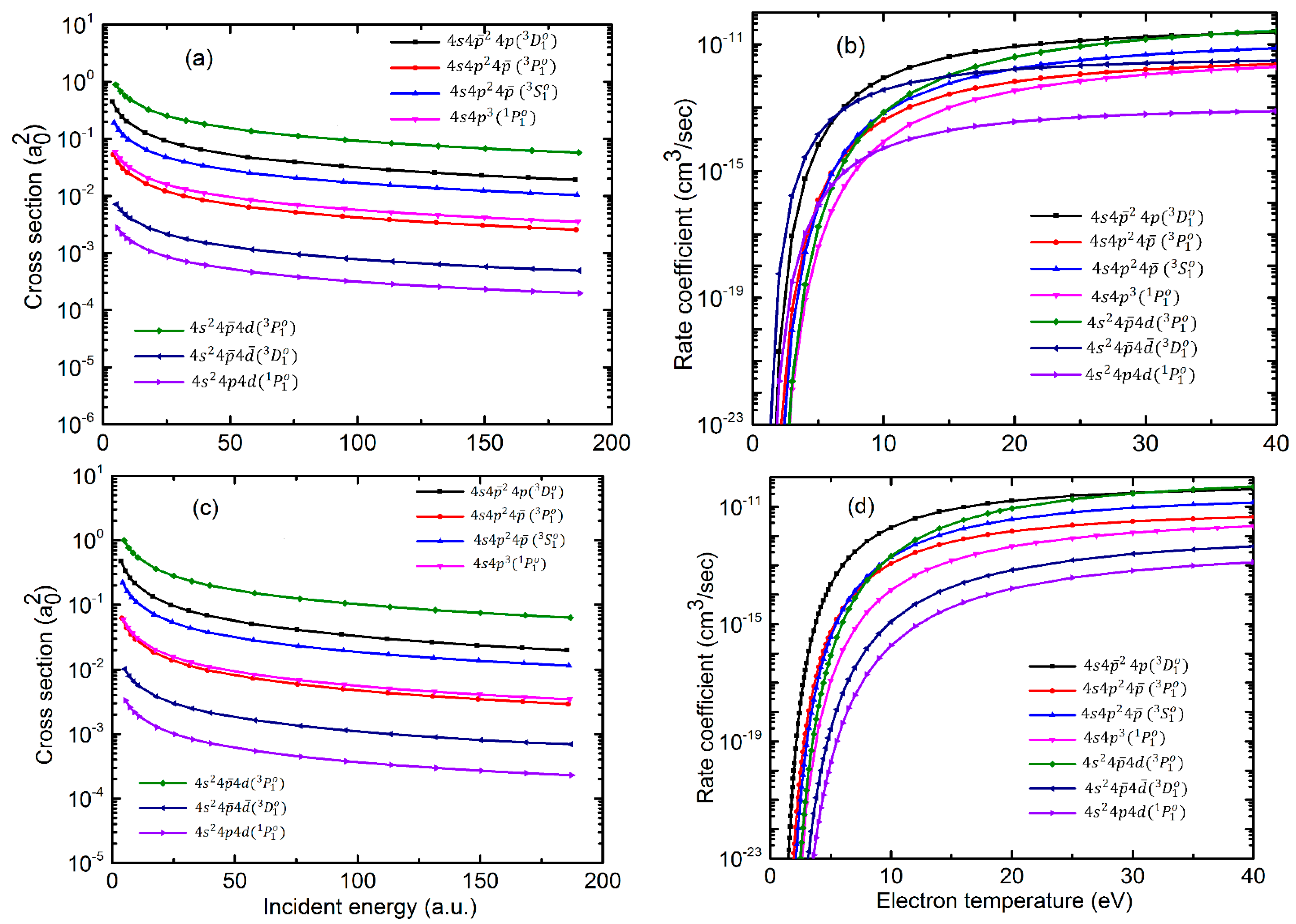
Atoms Free Full Text Electron Impact Excitation Of Ge Like Te20 Ndash Cd16 Ions Html